Effect of Sulfur Content in Wetar Pyrite Waste on the Formation Process of Sulfuric Acid Products at AIM Project Morowali
DOI:
https://doi.org/10.33096/jcpe.v10i1.1795Keywords:
AIM Project, Carbon Sulfur Analyzer, Pyrite Ore, Sulfuric Acid, Sulfur RecoveryAbstract
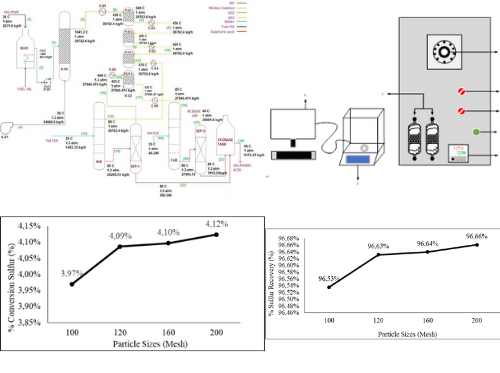
Pyrite ore is a sulfide mineral commonly found in mining operations, and has the potential to be converted into valuable chemical products. The substantial quantity of high-quality pyrite ore waste generated by the Wetar Copper Mine is non-reusable, potentially causing environmental issues upon exposure to air. To mitigate potential environmental contamination at the Wetar Copper Mine, the AIM Project was initiated to process high-quality pyrite ore waste into sulfuric acid. This study aims to determine the effect of sulfur content, measured as a percentage of sulfur recovery, on the conversion of SO2 gas to SO3. Concentrate and calcine samples with particle size variations of 100, 120, 160 and 200 mesh were analyzed on a Carbon Sulfur Analyzer (CSA) with combustion temperature variations of 1100, 1150, 1200 and 1250˚C to determine sulfur content as a reference for the potential amount of SO2 to SO3 conversion in sulfuric acid formation. The results showed that the conversion of SO₂ gas to SO₃ increased with increasing particle size, with a particle size of 200 mesh yielding an optimum sulfur gas conversion percentage of 4.12%. At the same conditions, a maximum sulfur recovery rate of 96.66% was obtained from an average particle size of 200 mesh.
Downloads
References
[1] S. Ma, M. Shi, C. Zhang, and Q. Cao, “Mineralogical Characteristics and Genetic Types of Pyrite with Different Occurrence : Constraints from Spectroscopy, Geochemistry and δ34S Stable Isotopes,” Minerals, vol. 14, pp. 1–14, 2023.
[2] B. Naglik, M. Slowik-Dumanska, T. Toboła, P. Derkowski, R. Habryn, and M. Markowiak, “Diversity of Pyrite-Hosted Solid Inclusions and Their Metallogenic Implications — A Case Study from the Myszków Mo–Cu–W Porphyry Deposit (the Kraków–Lubliniec Fault Zone, Poland),” Minerals, vol. 11, pp. 1–18, 2021.
[3] X. Gong et al., “Natural Pyrite as a Catalyst for a Fenton Reaction to Enhance Xanthate Degradation in Flotation Tailings Wastewater,” Minerals, vol. 13, pp. 1–12, 2023.
[4] A. A. Zahirah and A. E. Nur Afni Oktaviana, “The Effect of Impurities on Nickel Content In Ore Samples Using Oven and Roasted Preparation Methods,” Chem. Enviromental Enginering, vol. 1, pp. 117–125, 2025.
[5] H. Sun, M. Chen, L. Zou, R. Shu, and R. Ruan, “Study of the kinetics of pyrite oxidation under controlled redox potential,” Hydrometallurgy, vol. 155, pp. 13–19, 2015, doi: 10.1016/j.hydromet.2015.04.003.
[6] M. Jefferson et al., “Effect of pyrite textures and composition on flotation performance: A review,” Miner. Eng., vol. 201, no. February, pp. 1–15, 2023, doi: 10.1016/j.mineng.2023.108234.
[7] J. Zhang, Y. Yan, Z. Hu, X. Fan, and Y. Zheng, “Utilization of low-grade pyrite cinder for synthesis of microwave heating ceramics and their microwave deicing performance in dense-graded asphalt mixtures,” J. Clean. Prod., vol. 170, pp. 486–495, 2018, doi: 10.1016/j.jclepro.2017.09.175.
[8] J. Liu et al., “Characteristic and Geological Significance of Pyrite in Chang 73 Sub-Member in the Ordos Basin,” vol. 32, no. 12, 2021, doi: 10.11764/j.issn.1672-1926.2021.10.001.
[9] F. Ye, J. Liu, T. Xiong, and M. Xie, “Arsenopyrite removal from pyrite concentrate using pulsating high gradient magnetic separation,” Results Phys., vol. 10, no. August, pp. 822–826, 2018, doi: 10.1016/j.rinp.2018.08.008.
[10] T. Jiang et al., “A novel value-added utilization process for pyrite cinder: Selective recovery of Cu/Co and synthesis of iron phosphate,” Hydrometallurgy, vol. 193, no. February, p. 105314, 2020, doi: 10.1016/j.hydromet.2020.105314.
[11] W. Yao et al., “Dissociation mechanism of particulate matter containing arsenic and lead in smelting flue gas by pyrite,” J. Clean. Prod., vol. 259, p. 120875, 2020, doi: 10.1016/j.jclepro.2020.120875.
[12] J. G. Speight, Industrial Inorganic Chemistry, no. Chapter 3. United Kingdom: Matthew Deans, 2017.
[13] M. Alhanif, G. J. Sanyoto, and W. Widayat, “Process Integration of Sulfuric Acid Plant Based on Contact Process,” Front. Heat Mass Transf., vol. 15, 2020, doi: 10.5098/HMT.15.17.
[14] S. Sampat, “Sulphuric acid plant integration in a chemical complex,” Sulphuric Acid Technol. 399, no. March, pp. 1–5, 2022, [Online]. Available: www.sulphurmagaine.com.
[15] T. Mperiju et al., “Optimized Production of High Purity Sulphuric Acid via Contact Process,” Logist. Oper. Manag. Res., vol. 2, no. 1, pp. 1–13, 2023, doi: 10.31098/lomr.v2i1.1436.
[16] H. Qin, X. Guo, Q. Tian, D. Yu, and L. Zhang, “Recovery of gold from sulfide refractory gold ore: Oxidation roasting pretreatment and gold extraction,” Miner. Eng., vol. 164, no. January, p. 106822, 2021, doi: 10.1016/j.mineng.2021.106822.
[17] K. G. Thomas and A. P. Cole, Roasting Developments – Especially Oxygenated Roasting. Ken Thomas & Murray Pearson, 2016.
[18] B. S. Wardhana and A. As’ad Sonief, “Pengaruh Ukuran Partikel Terhadap Penyusutan Berat Dan Porositas Keramik Tanah Liat Dengan Aditif Onyx, Giok Dan Zeolit,” vol. IV, no. 1, pp. 5–9, 2018, [Online]. Available: http://jurnal.untirta.ac.id/index.php/jwl.
[19] D. Hari, T. Prasetiyo, A. Muhammad, I. Noor, and D. Kusuma, “Pengaruh Ukuran Mesh Terhadap Karakteristik Fisik Dan Kimia Biobriket Biji Kesambi,” vol. 7, pp. 368–378, 2023.
[20] B. Prameswara, “Analisis Pengaruh Variasi Jenis Batubara dalam Proses Aglomerasi Bijih Nikel Laterit Terhadap Kadar Ni dan Fe serta Morfologi Aglomerat Sebagai Bahan Umpan Mini Blast Furnace,” Institut Teknologi Sepuluh Nopember, 2017.
[21] J. B. Honkasalo, “Method for Obtaining Elemental Sulphur from Pyrite or Pyrite Concentrates,” 431,595, 1965.
[22] W. L. McCabe, J. C. Smith, and P. Harriot, Unit Operations of Chemical Engineering (5th Edition), 5th ed., vol. 136. Singapore: McGraw-Hill Companies, Inc., 1993.
[23] R. H. Perry, Perry’s Chemical Engineers’ Handbook 7th Edition, 7th ed., vol. 38, no. 02. North America: McGraw-Hill Companies, Inc., 2000.
[24] D. M. Himmelblau and J. B. Riggs, Basic Principles And Calculations in Chemical Engineering (8th Edition), 8th ed. Pearson Education, Inc., 2012.
[25] H. Müller, Sulfuric Acid and Sulfur Trioxide. Willey-VCH Verlag GmbH & Co., 2000.
[26] N. G. Ashar and K. R. Golwalkar, A Practical Guide to the Manufacture of Sulfuric Acid, Oleums, and Sulfonating Agents. New York, London: Springer Berlin Heidelberg, 2019.
[27] A. S. J. Wardhana and E. S. Damarwan, “Identification of Energy Saving Potential Through Energy Audit at PT. ABC,” J. Edukasi Elektro, vol. 7, no. 1, pp. 63–74, 2023, doi: 10.21831/jee.v7i1.61657.
[28] A. A. Ahiakwo and M. B. Nnah, “Effect of Mesh Count and Percentage Open Area on Throughput Capacity in Developing Cassava Cake Screening Medium,” vol. 30, no. 3, pp. 22–27, 2023, doi: 10.5455/nje.2023.30.03.04.
[29] Sufriadin et al., “Effects of particle sizes and roasting temperature on the Fe-Ni enrichment of limonite ore from the Wolo mine area , Southeast Sulawesi , using corncob char as reductant,” 2024, doi: 10.1088/1755-1315/1422/1/012007.
[30] A. Maulana, “Kajian Pertambangan: Studi Potensi Bahan Galian Bijih Emas (Au) Kabupaten Buol Tahun 2023,” Makassar, 2023.
[31] M. Hashemi, F. Pourfayaz, and M. Mehrpooya, “Energy, exergy, exergoeconomic and sensitivity analyses of modified Claus process in a gas refinery sulfur recovery unit,” J. Clean. Prod., vol. 220, pp. 1071–1087, 2019, doi: 10.1016/j.jclepro.2019.02.213.
[32] J. Sun, Z. Wang, and Z. He, “Indirect electrochemical leaching and separation of cobalt and lithium from spent LiCoO2 through recovery and reuse of sulfuric acid,” Sep. Purif. Technol., vol. 364, no. P3, p. 132587, 2025, doi: 10.1016/j.seppur.2025.132587.
[33] B. Koohestani, A. K. Darban, P. Mokhtari, E. Darezereshki, E. Yilmaz, and E. Yilmaz, “Influence of hydrofluoric acid leaching and roasting on mineralogical phase transformation of pyrite in sulfidic mine tailings,” Minerals, vol. 10, no. 6, pp. 1–15, 2020, doi: 10.3390/min10060513.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Journal of Chemical Process Engineering

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






