Evaluation of Electrocoagulation Process Efficiency in Laboratory Wastewater Treatment with Various Current Densities
DOI:
https://doi.org/10.33096/jcpe.v10i1.1533Keywords:
Electrocoagulation, laboratory wastewater, current density, COD, TSSAbstract
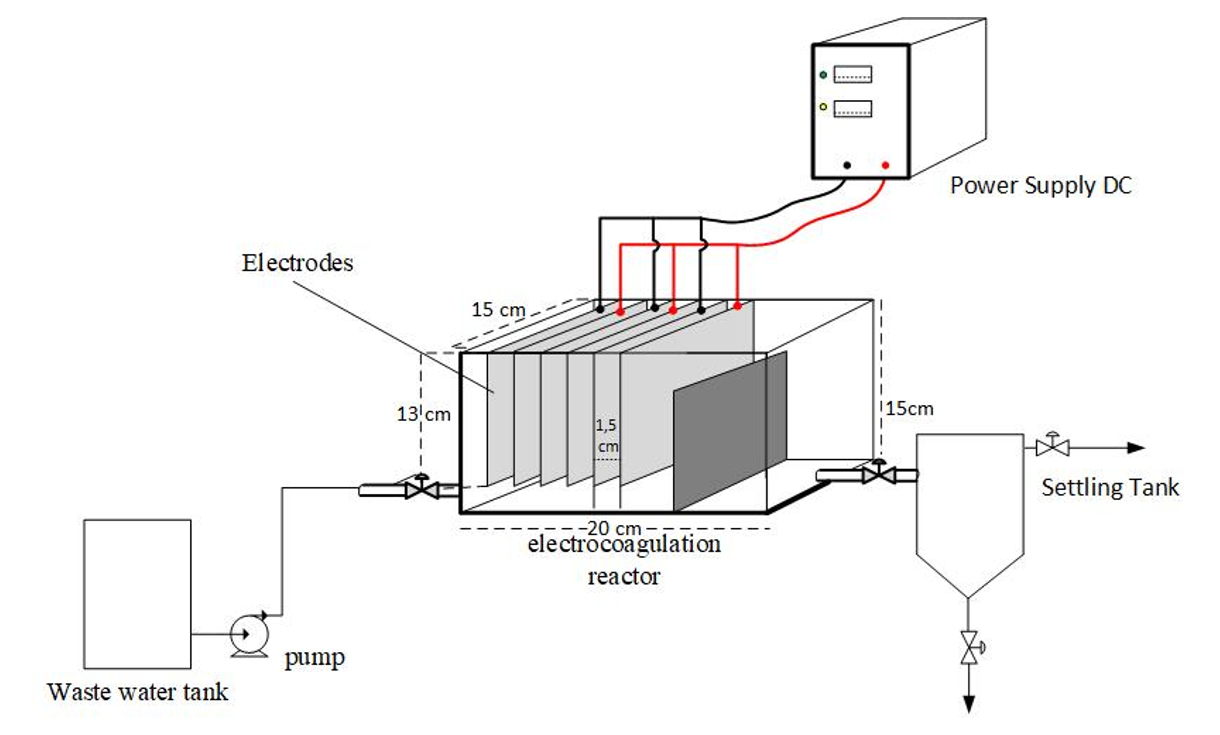
Laboratory wastewater contains harmful compounds such as COD, TSS, heavy metals, and toxic compounds that require treatment before being discharged into the environment. Laboratory wastewater contains various hazardous pollutants that can pollute the environment if not managed properly. This study aims to evaluate the efficiency of the electrocoagulation process in the treatment of laboratory wastewater of the Chemical Engineering Study Program of PGRI University Palembang with variations in current density and reaction time. The experimental method was used by designing an electrocoagulation reactor using aluminum electrodes. The independent variables tested include current density (76.92 A/m², 87.17 A/m², and 102.56 A/m²) and reaction time of 15, 30, 60 minutes. Parameters analyzed included COD, TSS, TDS, pH, electrode consumption, and energy consumption. The results showed that the electrocoagulation process effectively reduced pollutant parameters; at a current density of 102.56 A/m² for 60 minutes, COD decreased to 63.5 mg/L, TSS to 23.52 mg/L, pH increased to 8, and turbidity reduced to 20.48 NTU. The specific energy consumption reached 18.2 kWh/m³ with an operational cost of Rp27,300/m³. Based on the analysis, the optimal current density for laboratory wastewater treatment is 102.56 A/m² with a reaction time of 60 minutes. Electrocoagulation technology is recommended as an efficient, effective, and environmentally friendly treatment method for laboratory wastewater in higher education institutions.
Downloads
References
[1] N. Anggraini, T. E. Agustina, and F. Hadiah, “Pengaruh pH dalam Pengolahan Air Limbah Laboratorium Dengan Metode Adsorpsi untuk Penurunan Kadar Logam Berat Pb, Cu, dan Cd,” J. Ilmu Lingkung., vol. 20, no. 2, pp. 345–355, 2022, doi: 10.14710/jil.20.2.345-355.
[2] Y. Sukmawardani and V. Amalia, “Chemistry Laboratorium Liquid Waste Treatment Using Electrocoagulation Method,” J. Kartika Kim., vol. 2, no. 2, pp. 100–106, 2019, doi: 10.26874/jkk.v2i2.29.
[3] I. Nurhayati and S. Vigiani, “Penurunan Kadar Besi ( Fe ), Kromium ( Cr ), COD DAN BOD Limbah Cair Laboratorium Dengan Pengenceran ,” vol. 14, no. 1, pp. 74–87, 2020.
[4] M. Joning, L. Melawaty, R. Sira Sarungallo, and P. Studi Teknik Kimia, “Metode Elektrokoagulasi Untuk Pengolahan Limbah Cair Laboratorium Yang Mengandung Logam-Logam Berat Electrocoagulation Method For The Treatment Of Laboratory Wastewater Containing Heavy Metals,” Chem. Eng. J., vol. 1, no. 1, pp. 1–14, 2022.
[5] F. Fu and Q. Wang, “Removal of heavy metal ions from wastewaters: A review,” J. Environ. Manage., vol. 92, no. 3, pp. 407–418, 2011, doi: https://doi.org/10.1016/j.jenvman.2010.11.011.
[6] S. K. Gunatilake, “Methods of removing heavy metals from industrial wastewater. Multidiscip Eng Sci Stud 1: 12--18,” 2015.
[7] M. J. K. Ahmed and M. Ahmaruzzaman, “A review on potential usage of industrial waste materials for binding heavy metal ions from aqueous solutions,” J. Water Process Eng., vol. 10, pp. 39–47, 2016, doi: https://doi.org/10.1016/j.jwpe.2016.01.014.
[8] S. De Gisi, G. Lofrano, M. Grassi, and M. Notarnicola, “Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review,” Sustain. Mater. Technol., vol. 9, pp. 10–40, 2016, doi: https://doi.org/10.1016/j.susmat.2016.06.002.
[9] S. S. Hosseini, E. Bringas, N. R. Tan, I. Ortiz, M. Ghahramani, and M. A. Alaei Shahmirzadi, “Recent progress in development of high performance polymeric membranes and materials for metal plating wastewater treatment: A review,” J. Water Process Eng., vol. 9, pp. 78–110, 2016, doi: https://doi.org/10.1016/j.jwpe.2015.11.005.
[10] M. Guerreiro Crizel, T. M. Barreto, M. A. Fiori, G. L. Colpani, and J. M. Muneron de Mello, “Electrocoagulation Process as a Consolidated Technology in the Treatment of Industrial Effluents and as a Promising Process in the Treatment of Effluents Generated by Car Washes: A Brief Review,” ACS ES&T Water, vol. 4, no. 5, pp. 1978–2004, May 2024, doi: 10.1021/acsestwater.3c00413.
[11] D. A. N. T. Dan, “Electrochemistry: Electrochemical Cell, Thermodynamic and Kinetic Aspects,” J. Mar. Sci. Res. Oceanogr., vol. 5, no. 2, 2022, doi: 10.33140/jmsro.05.02.01.
[12] J. S. Al-Marri, A. B. Abouedwan, M. I. Ahmad, and N. Bensalah, “Electrocoagulation using aluminum electrodes as a sustainable and economic method for the removal of kinetic hydrate inhibitor (polyvinyl pyrrolidone) from produced wastewaters,” Front. Water, vol. 5, 2023, doi: 10.3389/frwa.2023.1305347.
[13] R. K.S, R. Anil, R. Varghese, S. Siby, E. A. Jose, and T. Thomas, “Solar-Powered Unit with Novel Reversible Inversion for Sustainable Electrocoagulation,” in 2024 7th International Conference on Circuit Power and Computing Technologies (ICCPCT), 2024, pp. 1199–1204. doi: 10.1109/ICCPCT61902.2024.10673224.
[14] M. Bharti, P. P. Das, and M. K. Purkait, “A review on the treatment of water and wastewater by electrocoagulation process: Advances and emerging applications,” J. Environ. Chem. Eng., vol. 11, no. 6, p. 111558, 2023, doi: https://doi.org/10.1016/j.jece.2023.111558.
[15] K. Wei et al., “Morphological engineering coupled with electronic engineering accelerates H2 production at the high current density,” Sep. Purif. Technol., vol. 326, p. 124814, 2023, doi: https://doi.org/10.1016/j.seppur.2023.124814.
[16] X. Lv et al., “Characterization of Bubble Behavior in Aluminum Reduction Cells BT - Light Metals 2016,” E. Williams, Ed., Cham: Springer International Publishing, 2016, pp. 347–352. doi: 10.1007/978-3-319-48251-4_57.
[17] N. Hajlaoui, I. Ksentini, M. Kotti, and L. Ben Mansour, “Experimental Study of Current Density and Liquid Phase Electric Conductivity Effects on Bubble Size Distribution in an Electroflotation Column,” Russ. J. Electrochem., vol. 55, no. 5, pp. 358–363, 2019, doi: 10.1134/S1023193519040025.
[18] R. S. Putra, M. Sarkawi, F. Faikha, and L. Fitmoko, “Analysis of Bubbles Size Produced in Electroflotation Using Graphite and Stainless Steel Electrode With DinoCapture 2.0,” in 2021 IEEE International Conference on Health, Instrumentation & Measurement, and Natural Sciences (InHeNce), 2021, pp. 1–5. doi: 10.1109/InHeNce52833.2021.9537230.
[19] Ş. İrdemez, Z. Bingül, S. Kul, F. Ekmekyapar Torun, and N. Demircioğlu, “The effect of pH on removal of phosphate from water using aluminum electrodes by electrocoagulation method,” El-Cezeri J. Sci. Eng., vol. 8, no. 3, pp. 1472–1479, 2021, doi: 10.31202/ecjse.948309.
[20] Y. Kwon, “A Study on pH Stability by Constant Current Control in Multi-stage Alkaline Water Ionizer TT - A Study on pH Stability by Constant Current Control in Multi-stage Alkaline Water Ionizer,” J. Inst. Electron. Inf. Eng., vol. 59, no. 10, pp. 121–128, 2022, doi: 10.5573/ieie.2022.59.10.121.
[21] F. Augusto Costa Mafra Passos, J. da Cruz Trajano de Souza, I. Daniel dos Santos, R. Neumann, P. Paulo Medeiros Ribeiro, and A. Junqueira Bourdot Dutra, “Effect of pH and current density on the physical properties of cobalt obtained by electrowinning from sulfate solutions,” Miner. Eng., vol. 211, p. 108697, 2024, doi: https://doi.org/10.1016/j.mineng.2024.108697.
[22] N. Galvão, J. B. de Souza, and C. M. de S. Vidal, “Landfill leachate treatment by electrocoagulation: Effects of current density and electrolysis time,” J. Environ. Chem. Eng., vol. 8, no. 5, p. 104368, 2020, doi: https://doi.org/10.1016/j.jece.2020.104368.
[23] S. U. Khan, M. Asif, F. Alam, N. A. Khan, and I. H. Farooqi, “Optimizing Fluoride Removal and Energy Consumption in a Batch Reactor Using Electrocoagulation: A Smart Treatment Technology BT - Smart Cities—Opportunities and Challenges,” S. Ahmed, S. M. Abbas, and H. Zia, Eds., Singapore: Springer Singapore, 2020, pp. 767–778.
[24] M. Ebba, P. Asaithambi, and E. Alemayehu, “Investigation on operating parameters and cost using an electrocoagulation process for wastewater treatment,” Appl. Water Sci., vol. 11, no. 11, p. 175, 2021, doi: 10.1007/s13201-021-01517-y.
[25] B. Nareerob and P. Jitto, “Effect of Current and Electrodes Area to Color Removal Efficiency and Energy Consumption by Electrocoagulation Process BT - Sustainable Development of Water and Environment,” H.-Y. Jeon, Ed., Cham: Springer International Publishing, 2021, pp. 169–179.
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Journal of Chemical Process Engineering

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.






